Hello to all the Chemistry enthusiasts reading this and welcome to my blog that is all about Quality Control Chemists! Please do consider that I am not an actual QC professional, and that this blog is written about an actual QC chemist that I met and interviewed with in order to gain insight into the field of quality control and its relations to high school chemistry.
Here's a little bit about this occupation and my agent of chemistry. Due to privacy issues, I will now be referring to my agent as "Joe" instead of releasing his real name.
Joe works for the company Javo-Mex, which is a manufacturer of soap, detergent, body wash, and more cleaning products which are then distributed to large cleaning companies such as P&G and Mr. Clean. He is the lead quality control chemist, and his job consists mostly of lab work, executing several chemical reactions and tests on either the raw materials or samples of products in the making. Many of these QC tests are actually very similar to the labs carried out at school, which will be explained more in further posts. As a QC chemist, Joe performs these tests and reactions in order to ensure that the raw materials or products meet all qualifications and standards set by the company, so that the products produced are of the highest quality and most importantly, safe to use. Joe also wanted me to mention that is job is completely different from what a chemical engineer does. While the engineer is part of the development, design, and processing of the product, Joe doesn't really have much say in that.
Joe's typical day at work consists of taking raw samples or samples of the products into the lab for analysis. He then follows different chemical methods such as titration, and using the HPLC to test the samples. If the sample does not meet the qualifications, then he must inform the supervisor in order to resolve the issue. Joe then fills out a sample report on the product or raw materials, and if the quality of the sample is poor, then the report is given to another department. If the sample is approved by Joe, then the product moves onto the packaging department, and approved raw materials are sent to the processing/manufacturing department to start production. A very important part of Joe's job is proper lab technique and safety, because he is working with hazardous chemicals frequently. This is similar to the importance of safety and proper lab practice in my school labs.
Monday 18 February 2013
Educational Background
Joe received an undergraduate degree in organic chemistry in China. He then went on to study in America, and got his master's degree in chemistry (concentrating in polymer chemistry) at the University of Detroit. He says that organic and inogranic chemistry, analytical chemistry, and physical chemistry were all extremely important courses in university that were vital to his career and field.
pH & Acidity/Alkalinity
An important quality control indicator is pH, which is important in both raw materials and midstage products. Joe checks the pH of raw materials or product samples daily before sending them off to the next stage. Besides modifying the pH of samples, it is also important that Joe does calibration checks on his pH meters by using standard buffer solutions (which, the pH is known) with a pH usually between 4 and 7. A pH meter is an essential piece of equipment in Joe's lab, and it is used to check the pH. I was so shocked when I heard him say that he uses a pH meter! I couldn't believe that a professional like him actually used equipment similar to what I use in my simple school labs. I guess there are a lot more similarities than I thought there would be.
Sodium hydroxide and hydrochloric acid are also common substances used by Joe to make adjustments to the pH. Therefore, they are known as pH buffers. Of course, NaOH is used to make the sample more basic, and HCl is used to make it more acidic. This is something that I am familiar with, as these are also two common chemicals we use in the lab. These two compounds are used to adjust the pH because they are strong bases and strong acids, and dissociate 100% in water, in order to maximize the amount of pH change. When a strong acid is used, hydronium ions (H3O + (aq)) are formed since the acid donates a proton to water, and the high levels of H3O+ cause the solution to be more acidic. When a strong base is used, many hydroxide ions will be formed in solution, thus raising the pH.
pH is important in the field of quality control, because there are specific, optimal ranges that the pH of specific products have to fall under. It's almost like how an enzyme works (but not quite)! When we were first introduced to the concepts of pH, acid, and base, we learned the approximate pH ranges of products, such as cleaning products (soap, detergent, etc.) having to be basic, and other commercial products like shampoo having to be slightly acidic. Well, this seemingly simple information is actually vital to Joe's field. I also learned that most cleaning products are basic because dirt often has a surface layer of carboxylic acid groups, and when placed in a basic solution (which they are soluble in due to the carboxylic acid's polar nature), they are netrualized to produce a carboxylic acid salt and water (something that I learned in organic chemistry!). Then the dirt is suspended into the water and is removed from the surface being cleaned.
Examples of netrualization of carboxylic acids with a strong base is shown above.
Sodium hydroxide and hydrochloric acid are also common substances used by Joe to make adjustments to the pH. Therefore, they are known as pH buffers. Of course, NaOH is used to make the sample more basic, and HCl is used to make it more acidic. This is something that I am familiar with, as these are also two common chemicals we use in the lab. These two compounds are used to adjust the pH because they are strong bases and strong acids, and dissociate 100% in water, in order to maximize the amount of pH change. When a strong acid is used, hydronium ions (H3O + (aq)) are formed since the acid donates a proton to water, and the high levels of H3O+ cause the solution to be more acidic. When a strong base is used, many hydroxide ions will be formed in solution, thus raising the pH.
Above is an illustration of sulphuric acid, a strong acid, forming a hydronium ion.
Examples of netrualization of carboxylic acids with a strong base is shown above.
Titration
Titration is a vital lab method used by Joe in his lab, because many of the products manufactured in the cleaning industry, such as detergent, are composed of an amalgamation of chemicals that do not directly contribute to the main usage of the product. This may include substances for fragrance, "padding/filling", and other additives. The usage of compounds for scent reminds me of esters, because they are known for giving off flavourful aromas, as shown in the esterification lab. A common "padding/filling" substance is soldium sulfate, which is cheap, and can be easily manufactured by reacting sodium chloride with sulphuric acid or sodium oxide with sulphuric acid. Also, it is a relatively stable compound that won't decompose, and it doesn't react with oxidizing or reducing agents at room temperature. Alright, on to titration.
Joe uses titration to check the percentage of "efficient chemicals" in product samples, and makes sure that it isn't too low or too high. An example of an "efficient chemical" in detergent would be an active ingredient such as sodium lauryl sulfonate. Just like in school labs, a titrant (chemical which the concentration is known) is used to quantitatively determine the concentration of a specific substance in the sample. Even though there is modern technology developed to perform the titration more efficiently, Joe still uses the traditional method --manually ading the titrant through a burette into the sample which has an indicator in it, and looking for the perfect colour change that indicates the endpoint.
One of the simpler titrations that Joe performs is an acid-base titration, which is similar to the ones I carried out in the lab last year. He performs this titration in order to determine the alkalinity of certain cleaning products that are basic. To do this, he adds the cleaning product into a beaker, and hydrochloric acid (the titrant) into the burette. An indicator (Joe actually does use phenolphthalein!) is added into the beaker as well. Joe adds HCl into the titrate until the endpoint, indicated by a faint pink colour in the beaker, is reached, reads the volume off the burette, and calculates the concentration of the cleaning product, making sure that it is at an appropriate level. This method is practically the same as the titrations I performed in school labs, except it is applied to a professional industry. Mole calculations, acid-base neutralizations, and concentration --concepts that I have studied intensely-- are all put to use here.
Structural formula of sodium sulphate is shown above.
One of the simpler titrations that Joe performs is an acid-base titration, which is similar to the ones I carried out in the lab last year. He performs this titration in order to determine the alkalinity of certain cleaning products that are basic. To do this, he adds the cleaning product into a beaker, and hydrochloric acid (the titrant) into the burette. An indicator (Joe actually does use phenolphthalein!) is added into the beaker as well. Joe adds HCl into the titrate until the endpoint, indicated by a faint pink colour in the beaker, is reached, reads the volume off the burette, and calculates the concentration of the cleaning product, making sure that it is at an appropriate level. This method is practically the same as the titrations I performed in school labs, except it is applied to a professional industry. Mole calculations, acid-base neutralizations, and concentration --concepts that I have studied intensely-- are all put to use here.
What a titration looks like. Except, at the endpoint, the colour of the titrate should be pale pink, not as intensly pink as the one shown above.
Viscosity
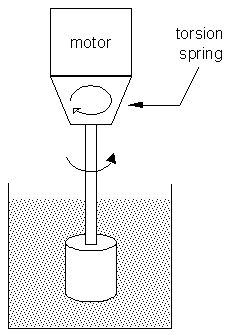
An important piece of equipment in Joe's lab is the viscometer, used to check the viscosity of the samples. Viscosity measurements can be used to determine the product's quality and efficiency, and this method is also very quick and reliable for analyzing product performance. By controlling the viscosity of products, and ensuring that they are in proper ranges, Joe enhances the quality and performance of the product as well as reduce production cost. There are many types of viscometers that Joe uses, such as a rotational viscometer, a capillary viscometer, and a vibro viscometer. A rotational viscometer works by inserting a cylindrical rotor into the sample and the rotor starts to rotate at constant speed. By using the principle that viscosity is directionally proportional to running torque, the twist angle caused by the torque is then proprtional to viscosity. This value is shown on the a meter on the viscometer.
Viscosity is a liquid's resistance to flow, but it is also dependent on the strength of intermolecular forces as well as the shape of the molecules. Usually, liquids that have higher intermolecular forces, which are polar molecules and/or can form hydrogen bonds, are more viscous than non-polar liquids. Sulfuric acid is a good example of this. It's intermolecular forces due to hydrogen bonding causes it to viscous, whereas an non-polar liquid with weak intermolecular forces like liquid nitrogen have low viscosity. A larger molecule may also be much more viscous, because of stronger dispersion forces as well as how relatively easy it is for the molecular chains to be "tangled" together. This is why long chained alkanes like fuel oil, and saturated fats are extremely viscous. Joe uses various substances to adjust the viscosity of products to a desired value. For instance, propylene glycol (1,2-propanediol) is used to increase the viscosity, due to its two hydroxyl groups which enable it to hydrogen bond, therefore increasing its intermolecular forces. Sodium xylene sulfonate is also used to lower the viscosity of cleaning products, because it is a hydrotrope. The concept of intermolecular forces that I learned in organic chemistry can be applied to viscosity which is vital to the chemistry of quality control in the cleaning industry.Viscosity is important to these products because of the importance of the texture and thickness of the liquid (like in shampoo and detergents) that feels nice to the hands, as well as produce a good amount of lather.
Above is an illustration of how a rotational viscometer works.
Above is the structure of propylene glycol.
Above is the structure of sodium xylene sulfonate.
Lab Safety and Hazards
Besides the qualitative, quantitative, and analytical chemical methods used in quality control, Joe must also be familiar with lab safety and be aware of potentially threatening hazards. Therefore, he must have proper lab technique and be able to handle the equipment and chemicals properly and safely. He must also wear goggles at all times, similar to when I am performing labs in school. Eye protection is vital when it comes to working in the lab. Joe must also wear gloves and a lab coat in order to prevent any hazardous staining, and physical contact with corrosive materials. Procedures that are dangerous such as highly exothermic reactions must be performed in the fume hood to minimize exposure to toxic fumes. This is similar to the usage of the fume hood in the evaporation and intermolecular forces lab, where I had to handle substances that gave off highly harmful fumes.
Joe must also safely dispose of chemicals (he never puts the sample that he tested on back into the source where he got it from!) depending on the type of waste, just like the simple hazardous waste bins we have at school. He also mentions that he must be very careful when handling highly concentrated sulphuric acid, which is a common catalyst in organic reactions, because it is a strong acid that is extremely corrosive and should be handled with great care. Material safety data sheets (MSDS) are also available to Joe and necessary to read, because they inform him on how to handle the materials properly to reduce risk. This is a real-world application of the MSDS that I used to filled out before school labs in grades 9 and 10. I didn't really enjoy doing that, but it was imperative.
Joe must also safely dispose of chemicals (he never puts the sample that he tested on back into the source where he got it from!) depending on the type of waste, just like the simple hazardous waste bins we have at school. He also mentions that he must be very careful when handling highly concentrated sulphuric acid, which is a common catalyst in organic reactions, because it is a strong acid that is extremely corrosive and should be handled with great care. Material safety data sheets (MSDS) are also available to Joe and necessary to read, because they inform him on how to handle the materials properly to reduce risk. This is a real-world application of the MSDS that I used to filled out before school labs in grades 9 and 10. I didn't really enjoy doing that, but it was imperative.
High-Performance Liquid Chromatography (HPLC)
High-performance liquid chromatography is a form of more advanced liquid chromatography that Joe uses commonly in the lab. He uses this method to separate compounds that are dissolved in a solution. With this, Joe can then figure out the chemical composition of the sample and if there are proper amounts of all the different compounds according to the formulations of the products.When the sample solution comes in contact with a particular solvent in liquid state, the different solutes in the solution interact with the solvent differently depending on several factors such as molecular size, absorption, ion-exchange, polarity, and more. These differences are what allow the components of the sample to be separated from each other. For instance, different polarities of the compounds in the solute (also depending on the polarity of the solvent) cause the different retention times, allowing the substances to be extracted and separated. This is due to "like dissolves like", causing polar to dissolve in polar, and non-polar to not dissolve in polar. Also, usually the smaller the molecular weight, the easier it is to travel through the solvent and therefore the difference in molecule sizes can cause separation.
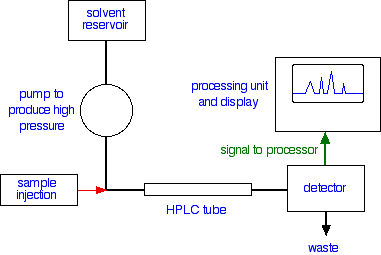
HPLC works differently than traditional simple chromatography, since it uses an HPLC instrument that consists of a resevoir mobile phase, a pump, an injector, a separation column, and a detector. The compound is separated by injecting the sample onto the column. The different moleules and ions in the sample pass through the column of the solvent at different rates due to many factors and characteristics as mentioned in the above paragraph. Here is more on how the HPLC instrument works in detail:
The liquid sample is introduced into a "loop" with a syringe. The sample will then travel through the column (also known as the HPLC tube), and the time it takes for the compound to travel through the column to the detector is know as the retention time. The retention time is based once again on characteristics like molecular size, polarity, temperature, etc. The substance will then reach the detector, and the detector detects the arrival of the substance with ultra-violet absorption. The UV detector reads how much light is absorbed by the chemical, and if it reads something, then that signifies the compound has passed through. The output is recorded as a series of peaks, and each peak represents a compound that was in the sample mixture. The peaks can be used to measure the quantity of the substances and determine more information.
What an HPLC instrument looks like.
The liquid sample is introduced into a "loop" with a syringe. The sample will then travel through the column (also known as the HPLC tube), and the time it takes for the compound to travel through the column to the detector is know as the retention time. The retention time is based once again on characteristics like molecular size, polarity, temperature, etc. The substance will then reach the detector, and the detector detects the arrival of the substance with ultra-violet absorption. The UV detector reads how much light is absorbed by the chemical, and if it reads something, then that signifies the compound has passed through. The output is recorded as a series of peaks, and each peak represents a compound that was in the sample mixture. The peaks can be used to measure the quantity of the substances and determine more information.
Schematic of an HPLC instrument.
Usage of Chemicals for Aromas
Different pigments and aroma chemicals are tested by and adjusted in the product by the QC chemist. All cleaning and hygiene products have these sort of "accessory" chemicals that provide the distinct scent or colour, such as green apple-scented and coloured detergent or coconut shampoo. It's also a vital selling point of the product. Compounds like aldehydes and benzene, which although are highly toxic and dangerous, provide a nice, sweet smell to products like detergents. Joe has to be careful when handling these chemicals, but he makes sure that there isn't too high of a concentration of these additives in the sample solution by using other methods such as titration HPLC, and colorimetry. Fatty aldehydes, which are long chains of carbon atoms that have a terminal aldehyde group are the types of aldehydes that are commonly used by QC chemists to adjust the fragrance of the product. Aromatic aldehydes such as benzaldehyde are also compounds that have a pleasant odour and used in Joe's industry. These compounds can be obtained from nature as raw materials, or they can be formed by oxidation of primary alcohols. However, Joe doesn't really perform the oxidation in the lab, as it takes time, and the quality/amount produced is not sufficient enough.
The usage of aromatics and aldehydes' scents to adjust the fragrance in QC reminds me of our study of esters and the specific esters that have a distinct scent used for different purposes in different industries. This was discovered through the esterification lab, and now I have found a place (quality control chemistry!) where these concepts can be applied in the real world.
The structure of benzaldehyde. Fatty aldehyde above.
The usage of aromatics and aldehydes' scents to adjust the fragrance in QC reminds me of our study of esters and the specific esters that have a distinct scent used for different purposes in different industries. This was discovered through the esterification lab, and now I have found a place (quality control chemistry!) where these concepts can be applied in the real world.
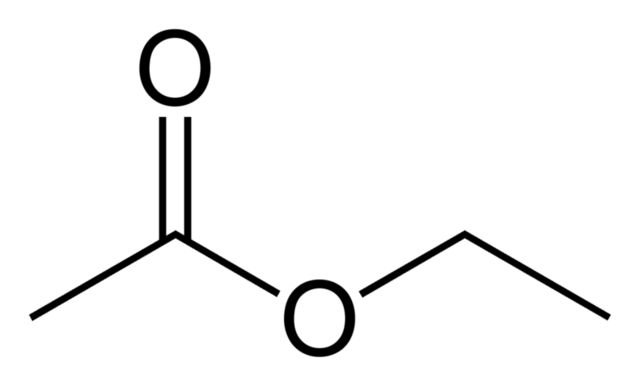
The structure of ethyl ethanoate is shown above, and it has a distinct "nail polish remover" scent.
Amlyase Test
Some enzymes are added to cleaning products like detergent in order to improve the washing performance and efficiency of the product. Two of the most common enzymes used in detergents that Joe utilizes are proteases and amylases, which are considered active ingredients, because they help break down stains faster and more easily. Amlyase is an enzyme that catalyzes the breakdown of starch into its monomers (this relates to not only catalysts and enzymes in biology, but also to polymer chemistry!). Ogliosaccharides, dextrins, and maltose, which are monomers of starch, are soluble, so it causes the stain to be physically cut off from the surface. Protease is similar to amylase, because it carries out hydrolysis in order to synthesize a large protein molecule by creating peptide bonds to join amino acids together. The proteins synthesized by protease are used to remove protein-based stains that are usually very difficult to get rid of.
Joe, the QC chemist, uses a variety of tests such as amylase test tablets to analyze the activity of amylase or other enzymes in the samples of cleaning products. The tablets are starch polymer chains that are interlinked to form spheres called bio-degradable starch microspheres (DSMs), which are insoluble in water. A water soluble blue dye is also added to the DSMs, but the whole thing still remains insoluble. The tablets are placed into the sample of the cleaning product being tested that has alpha-amylase in it, and the DSMs begin to be degraded by the enzyme. The blue dye will then be isolated from the DSM, and this is the key to measuring amylase activity levels. The dye can be measured in a spectrophotometer (it uses the measurement of reflection/transmission as a function of wavelength), and the concentration of the dye is proportional to amylase activity. Amylase levels must be in the right range to ensure that the product has enough active chemical in order to perform efficiently, but not too much to the point where the breakdown of starch is overpowering, causing other vital components of the detergent to be broken down as well. The use of enzyme as a catalyst and polymers/monomers is applied right here in quality control chemistry.
Above is part of a starch molecule that can be broken down into maltose monomers by hydrolysis, catalyzed by the enzyme amylase.
Here is a picture illustrating how DSM works. In this situation, DSM is used to stimulate blood clotting rather than indicate amylase activity levels. The blue dye is also evident in this picture.
Sodium Chloride & Soap Viscosity
When Joe is performing quality control tests on liquid soap, it is important for him to consider the viscosity of it because it is important for soap to have a certain thickness to it in order for the product to feel nice and comfortable on the consumer's hands. Joe increases the viscosity by adding sodium chloride --a table salt that I have come across many times in my career as a high school chemistry student. So how does it actually work?
First off, soap is prepared through saponification, since natural soaps are sodium or potassium salts of fatty acids. Saponification is the reaction that occurs between an ester and a strong base, such as sodium hydroxide or potassium hydroxide. When the reaction occurs, an alcohol and a salt of the acid is formed. The latter is the soap. For instance, when methyl methanoate reacts with sodium hydroxide, it forms methanol (the alcohol) and a sodium salt of ethanoic acid (the soap).
Sodium chloride can be added to the soap in order to precipitate it, thus making it more viscous. This is related to the common-ion effect, which is responsible for the reduction in solubility of a compound when a soluble compound consisting of one of the ions in the other compound is added to the solution in equilibrium. Therefore, adding sodium chloride to soaps causes it to precipitate, increasing the thickness. Not only is this due to the common-ion effect, but it is also due to increased ionic strength. Since sodium chloride is considered an electrolyte, it can be decomposed into sodium and chloride ions. Therefore there is an increase in ionic strength due to the formation of ions that causes precipitation. The concept of electrolytes, ions, and precipitation which I have learned throughout high school chemistry have been adapted to the adjustment of viscosity in soap in the field of quality control.
First off, soap is prepared through saponification, since natural soaps are sodium or potassium salts of fatty acids. Saponification is the reaction that occurs between an ester and a strong base, such as sodium hydroxide or potassium hydroxide. When the reaction occurs, an alcohol and a salt of the acid is formed. The latter is the soap. For instance, when methyl methanoate reacts with sodium hydroxide, it forms methanol (the alcohol) and a sodium salt of ethanoic acid (the soap).
The saponification of methyl methanoate in the presence of sodium hydroxide (first line of the image)
How NaCl is an electrolyte since it decomposes into Na+ and Cl- ions.
Colorimeter
The colorimeter is another one of Joe's essential lab equipment. It is a device used in colorimetry to determine the concentration of a solute in a solution that has colour by measuring the absorbance of specific wavelengths of light by the solution. With this device and concept, the Beer-Lambert law is applied. This law states that the concentration of a solute is directly proportional to its absorbance or colour intensity. However, there are many other factors that may affect the above statement. For instance, how intensely a substance absorbs colours may depend on the chemical being used, and its arrangement of electrons and energy levels. Since the concentration of a solute is proportional to its absorbance, then as concentration increases, the spectrum shown has fewer colours. Also, not only does the concentration of the solute affect the results, but so does the thickness of the solution. The absorbance is also directly proportional to the thickness of the substance. So overall, more intensely coloured compounds possess a more intense hue than less concentrated compounds. This was evident in the dilution lab that I did last year, where a series of liquid compounds were prepared through dilution, and each compound had a different concentration, evident by the varying colour intensities.
Joe uses the colorimeter by first selecting the appropriate wavelength, depending on the sample, to be measured, and then continues to carry out operating the machine to receive an absorbance value from the meter, and then this value can be used to find the concentration of the solution with Beer's Law.
The increasing intensity of hues (right to left) indicates increasing concentration.
A typical colorimeter.
The Interview
Here it is! A copy of the interview, questions and answers, I conducted with Joe!
What does a quality control chemist do?
- quality control: testing samples of raw materials or products either in mid-stage or close to final stage to make sure that they are of high enough quality, reaching the standards and qualifications, before the product can be mass produced
- research and development of products
- perform various chemical methods and procedures in the lab
Specifically, what company do you work for, what is your official title/position, and what are your duties?
- lead chemist: works in the lab, chemical reactions, testing the products, analyzing, quality control of raw materials to match all the qualifications/standards, quality control of final product, whereas the chemical engineer does the processing and development of the product
- company: Javo-Mex (soap/detergent/bodywash/handsoap/cleaning products company; manufacture for P&G, Mr. Clean)
Could you describe your typical day at work?
- take samples of raw materials or the finished products to the lab for analysis
- follow different chemical methods to do analysis to make sure samples have reached qualifications
- if not reached qualifications, must tell the supervisors and stop production to find the problem
- Inspect other co-workers
- fill out a sample report (raw materials, final products have different reports)
- if the raw material/final product/mid-stage product is poor: give the report to another department
- once final product is approved, send to packaging department and then they will ship it out
- once raw materials are approved, send to processing/manufacturing department to make the product
What is your work environment like?
- in a company laboratory
- must always be sanitary
- sometimes if there were a problem, had to go to manufacturing department
- go to warehouse to sample the raw material
- get finished products from the production/manufacture line
Who do you work with directly?
- director of the quality control department
- chemists, chemical engineers, mechanical engineers, electrical engineers (if manufacturing has problems, they may consult me, or chemist consults them)
What are some of the challenges you face at work and how do you overcome them?
- understanding all the chemistry, following the manufacturing/production rules and standards
- when something is wrong with the raw materials:
- if sample is not good, call the raw material manufacturing company, and send back the materials
-when something is wrong with the final products:
- if sample is not good, keep hold of product and find out the problem by contacting the different departments
- solving the problem is hard; meeting with different departments to solve the problem
- tracing back to the processing stages of product
Around how many hours do you work per day, and how many days per week?
- during the "high season" (summertime; higher demand) : overtime --> 10 hours or more
- regular season: 8 hrs
- during "slow season" : 8 hrs
What is your favourite part about being a chemical engineer?
- the chemistry knowledge you get to apply
- the technology
What is your least favourite part about being a chemical engineer?
- exposure to toxic/poisonous materials (not healthy for the workers)
- boring routine, doing same thing over and over, monotonous
What are your skills and attributes that make you fit for this job?
- knowledge of chemistry
- chemistry or chemical engineering degree
- communication
- attention to detail
Can you go over the QC chemist-specific technology you use?
- different types of instruments: HPLC (high performance liquid chromatography), Infrared spectrum, viscometer (specifically rotational), colorimeter, pH meter
- use computer analysis with HPLC
What are some of the "chemicals and substances" that you utilize and what are they used for?
- sodium hydroxide --> adjust pH, hydrochloric acid --> adjust pH, sodium chloride --> adjust the viscosity (for hand soap), different kinds of pigments to adjust fragrance and colour, various strong acids and bases, propylene glycol and sodium xylene sulfonate to adjust viscosity, indicators
Could you give me an example of the methods you use in quality control?
- for detergent: check the colour, smell the odour
- check the pH is in the range using pH meter: specific ranges for specific products
- specific gravity is in the range
- check the viscosity with viscometer
- wet chemical method/titration used to check percentage of efficient chemical and concentrations
- micro confirmation test: make sure no bacteria in the product
Can inform me about your educational background and credentials, such as what undergraduate degree you graduated with, master's degree, professional degrees, etc.?
- master's degree in chemistry (specifically in polymer) at University of Detroit
- undergrad degree in organic chemistry in China
Which chemistry courses did you find the most important for your career?
- organic/inorganic chemistry
- analytical chemistry
- physical chemistry
What kind of a chemistry foundation do you need for this career?
- good understanding of organic chemistry, and inorganic chemistry, math/calculations
What specific topics in chemistry does a QC chemist need a good grasp on?
- chemical news, mostly inorganic and organic chemistry
What kind of training is needed before starting the job?
- the company trains me
- specific to the company; must follow the company's methods and tests and their qualifications/standards
- lab skills, and certain methods were required before one could take the job
- familiarize with safety procedures and health hazards during the job
- proper lab technique was perfected with more practice, and consistently doing job
What are some of the health hazards in your workplace, and what are the safety procedures you need to know?
- wear goggles, do reactions in the fume hood, wear gloves, lab coat, safely dispose of chemicals (biohazardous waste)
- benzene, sulphuric acid had to be handled with care
- Material safety data sheets had to read
What do you think is the future for Quality Control Chemists?
- job demand will be strong; because many manufacturing companies need them to "make sure everything is correct"
- demand for high quality/safe products always high
- or else products will not be the right quality and potentially dangerous to the public
- high standards for cleaning/hygiene products calls for more QC chemists
How do you think your field will advance as technology advances?
- using more highly technological instruments rather than traditional lab techniques
- more efficient, more accurate, faster
- have to learn more new technology (computers, instruments)
- will become large part of career
Would you be able to tell me what your annual salary is around?
- varies depending on many factors
- $50,000-100,000
In-Service Training
The company, Javo-Mex, trained Joe and its employees to perform the quality control tests, methods, and procedures properly. The training was very "company-specific", because the company sets the standards and qualifications for the raw materials and products. Most of the training was done prior to the job, and also many lab skills such as operating the machinery and traditional chemistry lab methods were required. Safety procedures and health hazards were also gone over before Joe started, and he continued to familiarize himself with them as he worked in the environment. Proper lab technique and handling of equipment and substances were perfected through practice and constant use everyday. Communication with not only fellow chemists, but the supervisor and people from different departments was a skill that was sharpened as Joe continued doing his job.
Career History
Unfortunately, this will be a rather short post. Here's some general information on Joe's path to his current career as a QC chemist. He started off by obtaining his undergraduate degree in chemistry in China, he became a school teacher for a while. Then he went to America to work on his master's degree. After graduating, he was hired by Javo-Mex as a quality control chemist, and he worked his way up to lead chemist, which is his current position.
Acid-Base Titration Calculation
Here's a basic example of a calculation that would be done by a quality control chemist:
Click to view the pdf file that I have uploaded.
https://docs.google.com/viewer?a=v&pid=forums&srcid=MTYwODMxMjc5OTAwMjk2MTA5NDgBMTIzOTM0MjEzMjMyNDEyOTczMjUBUVQzMzMwNzlUZkVKATQBAXYy
Click to view the pdf file that I have uploaded.
https://docs.google.com/viewer?a=v&pid=forums&srcid=MTYwODMxMjc5OTAwMjk2MTA5NDgBMTIzOTM0MjEzMjMyNDEyOTczMjUBUVQzMzMwNzlUZkVKATQBAXYy
Potential for Advancement
Joe thinks that the demand for careers in quality control, especially QC chemists will rise in the future, because of the increasing demand of high quality, safe products. Manufacturing companies will have to rely heavily upon QC chemists to make sure the raw materials and products are at the standard, meet all qualifications, and that "everything is correct", as Joe states. This is a very important career in the manufacturing and processing industry that will not diminish due to strict standards placed on cleaning and hygiene products. A simple mistake like a slight pH shift may lead to potential harm towards the consumer and the public, which is why QC is vital.
This field will definitely advance as technology advances. More highly, technologically advanced instruments such as infrared spectrometers will be used over traditional chemistry methods like titrations and dilutions. Working with this type of technology may lead to more efficient, accurate, and faster work. However, people in this field will have to familiarize themselves with the newly developed technology that will become a large part of the career.
With experience and further training, QC chemists can further on move to supervisory, management, and policy research positions. Also, since many QC methods are very generic and "universal", there may be opportunities to move across sectors into an even higher position, or even move into a consultancy firm.
The annual salary ranges for different chemists, depending on which sector they are in, what company they work for, where they are located, and a lot more other factors. However, Joe provided me with the information that it usually ranges from $50,000 to as high as $100,000.
This field will definitely advance as technology advances. More highly, technologically advanced instruments such as infrared spectrometers will be used over traditional chemistry methods like titrations and dilutions. Working with this type of technology may lead to more efficient, accurate, and faster work. However, people in this field will have to familiarize themselves with the newly developed technology that will become a large part of the career.
With experience and further training, QC chemists can further on move to supervisory, management, and policy research positions. Also, since many QC methods are very generic and "universal", there may be opportunities to move across sectors into an even higher position, or even move into a consultancy firm.
The annual salary ranges for different chemists, depending on which sector they are in, what company they work for, where they are located, and a lot more other factors. However, Joe provided me with the information that it usually ranges from $50,000 to as high as $100,000.
Interesting Things about this Career
Subscribe to:
Posts (Atom)